In March 2004 the Committee on Safety of Medicines (CSM) advised that neither olanzapine nor risperidone should be used for the treatment of behavioural and psychological symptoms in dementia. They reported that use of these atypical antipsychotics was associated with a threefold increase in stroke in people with dementia and that there was a twofold increase in all-cause mortality with olanzapine in this group of patients. Risperidone could be used for acute psychosis in dementia but only on a short-term basis and under specialist advice. Olanzapine is not licensed for this use. The CSM also advised that history of and risks for cerebrovascular disease should be considered when prescribing these drugs.
A working group, including members from the Faculty for the Psychiatry of Old Age of the Royal College of Psychiatrists, the Royal College of General Practitioners, the British Geriatrics Society and the Alzheimer's Society, produced recommendations in light of the CSM guidance (Royal College of Psychiatrists, 2004).
Since the advice, a number of studies have investigated the risk of cerebrovascular incidents with these antipsychotics. Herrmann & Lanctot (Reference Herrmann and Lanctot2005) reassessed the data from 11 randomised controlled trials (RCTs) and found an increase in ‘ cerebrovascular adverse events’ but suggested that many were non-specific and not strokes. Another meta-analysis of RCTs reported a small increase in the risk of death compared with placebo (Reference Schneider, Dagerman and InselSchneider et al, 2005).
Two large retrospective population-based cohort studies of 32 000 and 11 000 patients respectively failed to find any increased risk of stroke (Reference Herrmann, Madami and LanctotHerrmann et al, 2004; Reference Gill, Rochon and HerrmannGill et al, 2005). However, a third large population-based retrospective study found an increased risk of cerebrovascular accidents with risperidone (but not with other atypicals) compared with traditional antipsychotics (Reference Percudani, Barbui and FortinoPercudani et al, 2005). Other studies have found no increased risk of cerebrovascular events with atypical antipsychotics (Reference Finkel, Kozma and LongFinkel et al, 2005; Reference Liperoti, Gambassi and LapaneLiperoti et al, 2005; Reference Moretti, Torre and AntonelloMoretti et al, 2005; Reference Suh and ShahSuh & Shah, 2005).
Mowat et al (Reference Mowat, Fowlie and MacEwan2004) argued that the CSM advice could prove detrimental to patient care. They advocated discussion with relatives and carers and argued that stroke, although serious, was a rare excess risk and demanded balanced thinking against other possible benefits of medication.
It was decided to study the response of doctors to the guidelines in Gateshead. A population-based sample was chosen in order to capture the response in primary as well as secondary care. This was done as part of an ongoing audit cycle.
Method
General practitioners (GPs) in Gateshead were contacted and asked for information on older patients who were on olanzapine or risperidone. Six practices were selected, thus sampling all the old age psychiatry sector teams. Data were initially gathered 10 weeks after the CSM guidance (Time 1). Psychiatric case notes were reviewed for those who had had contact with services. Care homes were also contacted for information. Diagnoses, reasons for prescriptions, other psychotropic medication and risk factors for cerebrovascular disease were obtained. We then ascertained whether they had been reviewed in light of the CSM guidance, by whom and the outcome of that review. A total of 98 patients were identified from the six practices. Two patients had recently died, leaving 96 patients. The deaths were not related to cerebrovascular disease. Data were again gathered on these patients 6 months later (Time 2) and included data from death certificates.
Results
Demographics of sample
The sample consisted of 27 men and 69 women with an age of 55-95 years (mean 80 years). Fifty-three lived at home, 10 in residential care, 10 in nursing care, 6 in elderly mentally infirm (EMI) residential care, 6 in EMI nursing care, 4 in elderly severely mentally infirm (ESMI) care, 1 in continuing care, 5 in respite care and 1 in palliative care.
By Time 2, 9 people had died and 5 had left the area, leaving 82 patients available for full follow-up. None of the deaths was related to cerebrovascular incidents.
Contact with psychiatric services
At Time 1, 43 patients were in contact with old age psychiatry services; 40 had been discharged from old age psychiatry services; 9 had not been seen by psychiatric services; 1 was an adult psychiatry patient; 1 had been discharged from adult services and 2 had been discharged from learning disability psychiatry services. At Time 2, 33 remained in contact with old age psychiatry services.
Diagnoses
Of those sampled, 52 people had a diagnosis of dementia,7 had other organic diagnoses and 35 had functional diagnoses. There were 2 others, 1 with schizoid personality traits and 1 with a personality dysfunction (Table 1).
Table 1. Diagnoses of 96 older patients who were prescribed olanzapine or risperidone
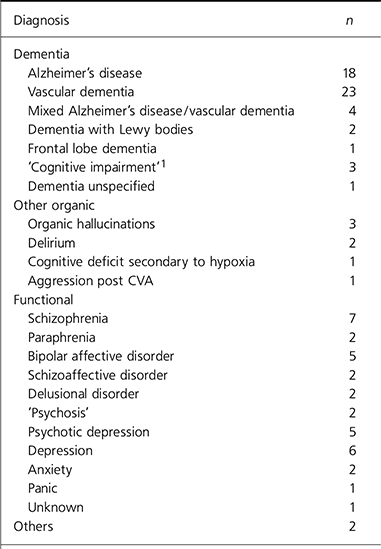
| Diagnosis | n |
|---|---|
| Dementia | |
| Alzheimer's disease | 18 |
| Vascular dementia | 23 |
| Mixed Alzheimer's disease/vascular dementia | 4 |
| Dementia with Lewy bodies | 2 |
| Frontal lobe dementia | 1 |
| ‘ Cognitive impairment’1 | 3 |
| Dementia unspecified | 1 |
| Other organic | |
| Organic hallucinations | 3 |
| Delirium | 2 |
| Cognitive deficit secondary to hypoxia | 1 |
| Aggression post CVA | 1 |
| Functional | |
| Schizophrenia | 7 |
| Paraphrenia | 2 |
| Bipolar affective disorder | 5 |
| Schizoaffective disorder | 2 |
| Delusional disorder | 2 |
| ‘ Psychosis’ | 2 |
| Psychotic depression | 5 |
| Depression | 6 |
| Anxiety | 2 |
| Panic | 1 |
| Unknown | 1 |
| Others | 2 |
Medication
Initially, 34 patients were on risperidone and 61 on olanzapine; 1 was on quetiapine but had been identified by the GP who was concerned about cerebrovascular risks. For olanzapine the most common total daily dose was 2.5 mg (range 2.5-20 mg); that for risperidone was 0.5 mg (range 0.5-2 mg). One patient was on a risperidone depot. The average time that people had spent on medication was 23.4 months (range 0-120 months).
There were 56 patients who were prescribed one or more other psychotropic medications. These included 30 who were receiving selective serotonin reuptake inhibitors, 7 on other antidepressants, 10 on cholinesterase inhibitors and others on mood stabilisers, benzodiazepines and hypnotics.
Cerebrovascular disease and risk factors
Of 52 people with dementia, 31 (60%) had cerebrovascular disease compared with 3 of 7 (43%) of those with other organic diagnoses and 9 of 35 (26%) with functional diagnoses. A further 16 people with functional diagnoses had other risk factors for cerebrovascular disease.
In the sample there were 5 patients who had had a cerebrovascular event while on olanzapine or risperidone prior to the CSM advice. Four had transient ischaemic attacks and one had a stroke. Four had dementia and all had been prescribed the medication for psychotic symptoms. Four of these patients had had previous cerebrovascular events prior to being on medication.
At Time 2 there had been no reports of further cerebrovascular events in any of the patients. None of the deaths during the study was related to cerebrovascular events.
Review of medication
At Time 1, 71 of 96 patients (74%) had been reviewed; 41 of 43 (95%) of those current to psychiatric services had been reviewed, 23 of 40 discharged patients (58%) and 5 of 9 (56%) of those not seen by psychiatric services. By Time 2, 90 of 96 patients (94%) had had their medication reviewed. This included 7 who had since died and 3 who had since left the area.
Initially 71 patients had been reviewed, 53 by old age psychiatry services. Of those, 41 were current patients; GPs had reviewed 17 and adult psychiatry services 1. Of the patients not currently in contact with old age psychiatry services but who had been reviewed by them, 2 had been referred again; others were reviewed by community psychiatric nurses or specific advice was given to the GP. At Time 2, most of the additional reviews had been performed by GPs. Another patient had been referred back to old age psychiatry services.
Outcomes
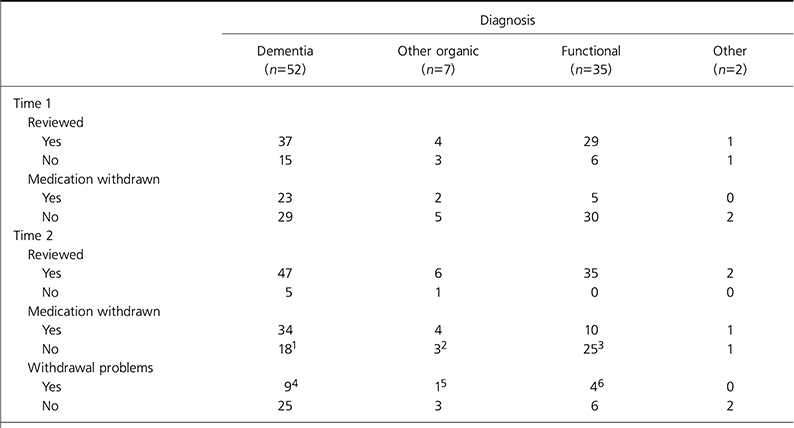
Outcomes for each group of patients are shown in Table 2.
Table 2. Data on 96 patients prescribed olanzapine or risperidone at 10 weeks after guidance (Time 1) and 6 months later (Time 2)
| Diagnosis | ||||
|---|---|---|---|---|
| Dementia (n=52) | Other organic (n=7) | Functional (n=35) | Other (n=2) | |
| Time 1 | ||||
| Reviewed | ||||
| Yes | 37 | 4 | 29 | 1 |
| No | 15 | 3 | 6 | 1 |
| Medication withdrawn | ||||
| Yes | 23 | 2 | 5 | 0 |
| No | 29 | 5 | 30 | 2 |
| Time 2 | ||||
| Reviewed | ||||
| Yes | 47 | 6 | 35 | 2 |
| No | 5 | 1 | 0 | 0 |
| Medication withdrawn | ||||
| Yes | 34 | 4 | 10 | 1 |
| No | 181 | 32 | 253 | 1 |
| Withdrawal problems | ||||
| Yes | 94 | 15 | 46 | 0 |
| No | 25 | 3 | 6 | 2 |
Discussion
The CSM advice has changed the management of the majority of patients with dementia. Our study found that management was changed in a smaller proportion of patients with functional illness to whom the advice applied than patients with dementia. From the outset, many clinicians were making risk-benefit decisions not to withdraw medication. In many cases these were prompted by previous trials of withdrawing medication that had failed. For the most part, these decisions were well documented and involved discussions with patients, where appropriate, and their families. This practice is in line with the subsequent guidance issued by the Faculty for the Psychiatry of Old Age in November 2004. The workload of old age psychiatry teams was increased by the CSM advice through extra reviews and giving advice to GPs, but there were fewer re-referrals than might have been expected.
The numbers of reported cerebrovascular events on medication were low. There were five reported events prior to the advice and none over the 8 months of the study. This agrees with the findings from most of the larger population-based studies (Reference Herrmann, Madami and LanctotHerrmann et al, 2004; Reference Gill, Rochon and HerrmannGill et al, 2005). It should be noted, however, that this study was not designed to specifically investigate the occurrence of cerebrovascular events.
There was a high rate of significant problems associated with withdrawal of medication. In over a third of these cases, the eventual outcome was a return to the original antipsychotic following risk-benefit discussions with patients, relatives and carers.
A limitation of this relatively small study is that it only included data on patients who were already on olanzapine or risperidone. It did not investigate the effect of the guidance on new prescribing, which one would anticipate to be more dramatic.
Strengths of the study are that it gathered information from both primary and secondary care, included patients with a range of diagnoses and in following their progress over 8 months it investigated the initial response to the guidance and subsequent problems.
In conclusion, the CSM guidance on the prescription of atypical antipsychotics for older adults has changed practice for existing patients but with a high rate of clinical problems and numerous patients remaining on, or returning to, olanzapine or risperidone.
Declaration of interest
None.
Acknowledgements
I thank Ann Emmerton, Pat Welsh, Pam Currey, Catherine Bell, Richard Harrison, Peter Thompson, Pam Stephenson, Apsara Pannikar and Alan Thomas from the Department of Old Age Psychiatry, Bensham Hospital.







eLetters
No eLetters have been published for this article.