Many people with psychotic illnesses respond poorly to the medication regimens in published guidelines which, however worthy, are often of limited help in ‘real world settings’. This is perhaps one reason why combination antipsychotic therapy and/or high-dose antipsychotic treatment are common in clinical practice despite published recommendations and lack of research evidence. The Royal College of Psychiatrists’ consensus statement on high-dose antipsychotic medication defines it as ‘a total daily dose of a single antipsychotic which exceeds the upper limit stated in the British National Formulary (BNF) […] or a total daily dose of two or more antipsychotics which exceeds the BNF maximum using the percentage method’. 1 Similarly, combination antipsychotic therapy is defined as ‘the concurrent use of two or more antipsychotics’. 1
There are published reports of combination and high-dose antipsychotic treatment from a number of countries. For example, an audit of 43000 psychiatric in-patients in the UK found that 48% of patients were on combined antipsychotic therapy and 20% on high-dose antipsychotics, Reference Harrington, Lelliott, Paton, Okocha, Duffett and Sensky2 and an Australian study of out-patients with schizophrenia showed a 13% rate of combination antipsychotic therapy. Reference Keks, Alston, Hope, Krapivensky, Culhane and Tanaghow3 Japanese studies reported that combined antipsychotics were used in 490% of patients Reference Ito, Kubota and Sato4 and a prevalence of 69% in 2005. Reference Ito, Koyama and Higuchi5 Studies of clinical practice in Scotland found a prevalence of 30% for combined antipsychotic treatment, Reference Taylor, Shajahan and Lawrie6 whereas a report from Wales found prevalence rates of 67.3% for combined therapy and 13.7% for high-dose antipsychotic treatment. Reference Tungaraza, Gupta, Jones, Poole and Slegg7 More recently, a study from London, UK, reported prevalence rates of 10% and 55% for combined and high-dose antipsychotic treatment respectively. Reference Grech and Taylor8
Although combined antipsychotics may increase adverse drug effects, some have argued that certain antipsychotic combinations may have benefits; Reference Grech and Taylor8,Reference Taylor9 for example, adding aripiprazole to clozapine may help some patients to lose weight and improve metabolic abnormalities. Reference Fleischhacker, Heikkinen, Olie, Landsberg, Dwaele and McQuade10 Similarly, co-therapy with aripiprazole and haloperidol has been reported to help normalise prolactin levels in patients treated with haloperidol alone. Reference Shim, Shin, Kelly, Jung, Seo and Liu11 One polypharmacy approach commonly used is clozapine augmentation which has been reported to improve positive and negative symptoms. Reference Josiassen, Joseph, Kohegyi, Stokes, Dadvand and Paing12-Reference Langan and Shajahan14
Guidance
To promote good practice of combination/high-dose antipsychotic treatment, a number of guidelines have been published. In the UK, the National Institute for Health and Care Excellence (NICE) states ‘do not initiate regular combined antipsychotic medication, except for short periods (for example, when changing medication)’, 15 and BNF guidance 16 does not recommend prescribing more than one antipsychotic at the same time. The Royal College of Psychiatrists’ consensus statement comments that exceeding the recommended antipsychotic dose range ‘is likely to exceed the acceptable risk-benefit ratio’, 1 and the Maudsley Prescribing Guidelines recommend that ‘the use of high-dose antipsychotics should be an exceptional clinical practice and only ever employed when standard treatments, including clozapine, have failed’. Reference Taylor, Paton and Kapur17
On the back of these national guidelines, local policies have been developed throughout the UK; for example, in Glasgow the high-dose antipsychotic therapy guidelines recommend that ‘combination antipsychotic therapy should only be used as part of a considered treatment plan’. 18 Given these treatment recommendations, we were interested in the question: how does actual prescribing practice in a community setting compare with these treatment recommendations?
The aim of the present study was to assess prescribing practice for a sample of patients with psychotic illness treated by a community mental health team based in West Glasgow (Arndale Mental Health Resource Centre) and compare this with published guidelines and findings from other centres.
Method
The Arndale Mental Health Resource Centre is located in Drumchapel, in West Glasgow. The catchment area of the resource centre encompasses a population from a wide spectrum of Scottish Index of Multiple Deprivation (SIMD). The centre has a case-load of about 700 adults with mental health problems. Gartnavel Royal Hospital provides in-patient care for patients from this catchment. Patients attending the Arndale Mental Health Resource Centre with ICD-10 19 F20-F29 diagnoses were identified using the NHS Greater Glasgow and Clyde Health Board patient information management system (PiMS). Information on demographics, diagnosis, age at onset, duration of illness, treatment prescribed, rationale for combination/high-dose antipsychotic treatment was collected for this cohort from case notes, prescription sheets and staff. We recorded details of antipsychotic prescription, route of administration, daily dose, combination treatment and high-dose prescription, documentation in relation to rationale and informed consent for combined/high-dose treatment, and information about recommended monitoring of these patients.
We defined combined antipsychotic therapy (CAT) operationally as the concurrent use of 41 antipsychotic for 43 months and high-dose antipsychotic treatment (HDAT) 4100% BNF daily dose, using aggregate percentages. 18,Reference Yorston and Pinney20,Reference Hayhurst21 The main audit standards were derived from existing guidelines: 1,15,18
-
1 combination antipsychotic treatment should be no more than 10% of the total of patients receiving antipsychotics
-
2 high-dose antipsychotic treatment should be no more than 5% of the total of patients receiving antipsychotics
-
3 all patients on combined/high-dose antipsychotic treatment should have information in their health record regarding rationale and consent for this treatment
-
4 all patients receiving high-dose antipsychotics should be identified and monitored as per recommended guidance.
Results of the first audit cycle (audit 1) with recommendations were presented in a structured format using a PowerPoint presentation to multidisciplinary staff at the Arndale Mental Health Resource Centre (including medical staff members) as part of our monthly lunch-time team teaching session to increase staff awareness. Patients on combined/high-dose antipsychotic treatment were subsequently identified using their unique PiMS number and the nurse team leader was responsible for prioritising these patients for an early multidisciplinary review using an integrated care pathway. 22 Results were also presented in the same format at Gartnavel Royal Hospital as part of the internal teaching programme and as a poster at the NHS Scotland Event in Glasgow. The audit was repeated after 1 year (audit 2), gathering similar information as in the first cycle to identify any change in practice. The results of the completed audit were presented at different venues as part of advanced trainees’ teaching in Glasgow, the National Audit Conference in Edinburgh and as a poster at the 2012 Royal College of Psychiatrists’ International Congress in Liverpool.
Statistical analysis
There were 135 patients in audit 1 and 150 patients in audit 2 (135 patients from audit 1 plus an additional 15 patients). For statistical analysis, these data cannot be considered as two independent samples. One approach in such a situation would be to impute the missing data (e.g. when baseline values in a clinical trial are carried forward for missing follow-up data). However, the data in this study were missing from the first and not the second audit, so there is not a logical way to impute this. The only statistical approach would therefore be to use paired methods of analysis and to compare categorical data using McNemar’s chi-squared test.
Results are presented for each audit descriptively for comparison purposes. Since 135 of the patients were followed up, statistical analysis was done using McNemar’s chi-squared test, a non-parametric test used in paired samples after removing the results for the additional 15 patients in audit 2.
Results
Audit 1
A total of 150 patients meeting entry criteria were identified; 8 were excluded (n = 4 F33 diagnosis; n = 3 transferred elsewhere; n = 1 deceased). Out of 142 cases, information was gathered on 135 patients; 72.6% were male and 27.4% were female (7 case notes were unavailable at the time of audit). Patients’ age ranged from 24 to 74 years (mean 46 years) in the HDAT group compared with 29-70 years (mean 45 years) in the CAT group. Mean duration of illness was 18 years (range 1-48) in the HDAT group and 20 years (range 2-38) in the CAT group. At the time of the study, 98.5% of the patients lived in the community and 1.5% were in-patients. Most patients had a diagnosis of schizophrenia (ICD-10 code F20, n = 94): other diagnoses included schizoaffective disorder (F25, n = 21), persistent delusional disorders (F22, n = 10), schizotypal disorder (F21, n = 7), and acute and transient psychotic disorders (F23, n = 3).
Table 1 shows that 88% (n = 118/135) of the population studied received high-dose antipsychotics and 12% (n = 16/135) were on combination antipsychotic treatment; this is 2% in excess of the standard set at the onset. Of the total sample, 3.7% (n = 5/135) (but 31% (n = 5/16) receiving combined antipsychotics) received high-dose antipsychotic treatment, meeting the standard originally set. Ten per cent of the total sample had 100% BNF daily dose (most of these patients were on olanzapine 20 mg/day). Of the CAT group, 38% were prescribed an anticholinergic, as opposed to 21% of the HDAT group. Eighty per cent of the total sample received oral medication (Table 2), 32% were on depot medication (Table 3) and 12% were on combined antipsychotic therapy. The most common combination was a depot antipsychotic with oral chlorpromazine or haloperidol. The most frequently used drug was clozapine (22%), followed by amisulpride (15%) and olanzapine (14%). The preferred drug for high-dose therapy was clozapine (21%) and for combination treatment, amisulpride (50%).
The rationale for combined antipsychotic therapy was described in 69% (11/16) of patients; the most common reason was to improve symptom control. In the remaining five cases, there was no recorded evidence of discussion about combination treatment. Forty per cent (2/5) of patients on high-dose antipsychotics had recorded evidence of monitoring in relation to blood tests, electrocardiogram (ECG) and other physical health checks.
Practice recommendations after audit 1 were the following:
-
• regular updating of information, including transfer, discharge and change in ICD-10 diagnosis
-
• clear recording of current antipsychotic drug treatment with reason for, and duration of, high-dose/combined antipsychotic treatment
-
• in patients on combination antipsychotic treatment, regular review to justify its use; reduce if possible
-
• in patients on high-dose antipsychotics, regular review to justify its use; reduce if possible
-
• review and improve monitoring of patients on combined/high-dose antipsychotics using an integrated care pathway to facilitate this process. 22
Audit 2
In total, 156 patients were identified; 6 individuals were excluded (n = 1 F33 diagnosis; n = 5 discharged or transferred to other areas), resulting in 150 patients included in audit 2. Of the sample, 75% was male and 25% was female. The age range for the HDAT group was 24-76 years (mean 48 years) compared with 33-71 years (mean 46 years) in the CAT group. In this audit, 98% of patients lived in the community and 2% were in-patients.
In this cycle, 10% (n = 15/150) of patients received combined antipsychotic therapy compared with 12% (n = 16/135) in audit 1 (McNemar’s P = 0.25 for 12% v. 9.6% on n = 135 paired samples). Two per cent (n = 3/150) of the sample received high-dose antipsychotics in comparison to 3.7% (n = 5/135) in audit 1 (McNemar’s P = 0.50 for 3.7% v. 2.2% on n = 135 paired samples). Among the additional 15 patients in audit 2, 2 patients were on combined antipsychotic treatment and none received high-dose antipsychotics. In 87% (n = 13/15) of the CAT group, there was better recording of rationale, discussion with the patient, medication update and monitoring of blood tests; this was achieved by giving priority to reviewing patients on combined/high-dose antipsychotics using an integrated care pathway. 22
Prescription patterns of antipsychotic medication remained much the same and, again, clozapine was the most commonly prescribed medication, followed by amisulpride and olanzapine. Prescription of aripiprazole had increased from 3 to 7 patients and that of chlorpromazine was reduced by 30%, from 11 to 8 patients.
We would argue that removing three patients from the CAT group and two patients from the HDAT group is clinically significant as combination therapy is the main reason for high-dose antipsychotic treatment, and the latter can cause serious harm to patients, including cardiac toxicity and sudden death. This was achieved by targeted enhanced review and monitoring of patients on combined and high-dose antipsychotics using an integrated care pathway. We looked for differences in the recording of patients’ monitoring when receiving any antipsychotic treatment in both audits. The results were 1.5% (n = 2/135) in audit 1 and 10.6% (n = 16/150) in audit 2 (McNemar’s P50.001 for 1.5% v. 12.0% on n = 135 paired samples). In audit 1, 40% (n = 2/5) of the HDAT group were monitored and in audit 2, 67% (n = 2/3) were monitored.
Clozapine was the favoured antipsychotic medication for high-dose antipsychotic treatment, followed by amisulpride and olanzapine in both audits, consistent with other reports of the use of clozapine in chronic and treatment-resistant cases of schizophrenia. Reference Langan and Shajahan14,15,22,Reference Howes, Vergunst, Gee, McGuire, Kapur and Taylor23 Prescription of aripiprazole increased by more than double in 1 year and was more commonly used in patients who were mentally stable but experienced side-effects, especially weight gain. It was occasionally used as an adjunct to clozapine, as evidence suggests helping patients to lose weight and to improve other metabolic parameters. Reference Langan and Shajahan14 The prevalence of chlorpromazine or haloperidol prescription, in addition to depot medication, for agitation or insomnia fell by 30% in audit 2.
Discussion
Several audits over the past two decades have highlighted the growing prevalence of combination and high-dose antipsychotic treatment and the significant variation of recommended practice has been noted by various authors. Reference Harrington, Lelliott, Paton, Okocha, Duffett and Sensky2,Reference Ito, Koyama and Higuchi5-Reference Taylor9,Reference Langan and Shajahan14,Reference Hayhurst21-Reference Tyson, Mortimer and Wheeler25 However, there have been few community-based studies in naturalistic populations of patients. The few published studies have included highly selective patient groups that may not reflect ‘real-world’ practice. Reference Harrington, Lelliott, Paton, Okocha, Duffett and Sensky2,Reference Ito, Kubota and Sato4,Reference Ito, Koyama and Higuchi5,Reference Hayhurst21,Reference Paton, Barnes, Cavanagh, Taylor and Lelliott24,Reference Tyson, Mortimer and Wheeler25 In the current study, we included all patients with psychotic disorders in one locality in order to try to ensure a representative sample. Encouragingly, the majority of patients (88-90%) received high-dose antipsychotics, a significant proportion (80%) received oral medication and about a third (32%) were on depot medication.
Table 1 Number of patients receiving high-dose and combination antipsychotics

|
British National Formulary
dose, % |
Patients receiving one antipsychotic, % (n) (n = 118) |
Patients receiving combination antipsychotics, % (n) (n = 16) |
Total patients,Footnote
a
% (n) (n = 135) |
|---|---|---|---|
| <100 | 89 (105) | 69 (11) | 87 (116) |
| 100 | 11 (13) | 0 (0) | 10 (13) |
| >100 | 0 (0) | 31 (5) | 3.7 (5) |
a. One patient was taking no medication.
Table 2 Patients treated with oral antipsychotic medication

| Drug | Patients, n | Dose, mg/day: mean (range) |
|---|---|---|
| Clozapine | 30 | 443 (125-900) |
| Amisulpride | 20 | 550 (200-1200) |
| Olanzapine | 19 | 13 (7.5-20) |
| Risperidone | 11 | 4 (1-8) |
| Chlorpromazine | 11 | 305 (50-850) |
| Quetiapine | 5 | 365 (75-600) |
| Haloperidol | 4 | 13 (10-15) |
| Aripiprazole | 3 | 13 (5-20) |
| Sulpiride | 2 | 400 (0-400) |
| Flupentixol | 1 | 6 (0-6) |
| Zuclopenthixol | 1 | 20 (0-20) |
| Total | 107 (80%) |
The evidence base for high-dose antipsychotic treatment as a therapeutic strategy is not strong but there is support for its use in treatment-resistant schizophrenia. Reference Grech and Taylor8,Reference Langan and Shajahan14,Reference Taylor, Paton and Kapur17,22 In our study, almost all patients on high-dose antipsychotics had a diagnosis of treatment-resistant schizophrenia, highlighting a need for national and local guidelines for patients with schizophrenia who fall into this subgroup but who have difficulties with medication adherence and/or refuse blood monitoring.
Table 3 Patients treated with depot antipsychotic medication
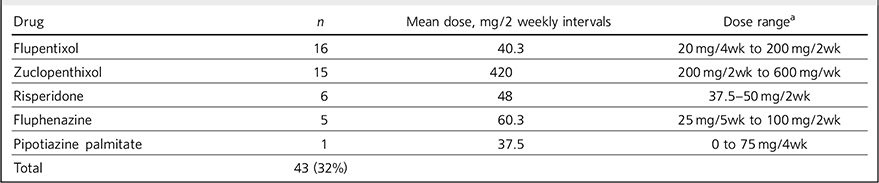
| Drug | n | Mean dose, mg/2 weekly intervals | Dose rangeFootnote a |
|---|---|---|---|
| Flupentixol | 16 | 40.3 | 20 mg/4wk to 200 mg/2wk |
| Zuclopenthixol | 15 | 420 | 200 mg/2wk to 600 mg/wk |
| Risperidone | 6 | 48 | 37.5-50 mg/2wk |
| Fluphenazine | 5 | 60.3 | 25 mg/5wk to 100 mg/2wk |
| Pipotiazine palmitate | 1 | 37.5 | 0 to 75 mg/4wk |
| Total | 43 (32%) |
a. nwk = number of weekly intervals.
Compared with the results of other published audits, including one community-based study, Reference Tungaraza, Gupta, Jones, Poole and Slegg7 our results show relatively lower prevalence rates of combination and high-dose antipsychotic treatment in both audit cycles. However, despite the widespread use of second-generation antipsychotics, there has been no reduction in the use of combination or high-dose antipsychotic treatment. This is perhaps not surprising given that first- and second-generation antipsychotics have similar efficacy. Reference Geddes, Freemantle, Harrison and Bebbington26
Schizophrenia is associated with increased mortality which is not explained by an increased suicide rate alone. Reference Langan and Shajahan14,22,Reference Waddington, Youssef and Kinsella27 Patients with schizophrenia are at higher risk of cardiovascular morbidity and mortality and the development of diabetes mellitus and metabolic syndrome. Reference Langan and Shajahan14,Reference Curkendall, Mo, Glasser, Rose Stang and Jones28 In this audit, physical health monitoring of patients prescribed with combination and high-dose antipsychotics improved from 40% to 67%, but this is still not satisfactory as many patients did not receive, or did not have any record of having received, monitoring as per published recommendations. If levels of high-dose antipsychotics and combination antipsychotics are higher than detected in this audit, due to discretionary self-medication, Reference Barnes and Paton29 then it is likely that many more patients will require physical health monitoring. An integrated care pathway could be a beneficial tool in patient monitoring. 22 An integrated care pathway is a multidisciplinary outline of anticipated care, placed in an appropriate time frame to help a patient with a specific condition (e.g. schizophrenia, depression, dementia) move progressively through a clinical experience to positive outcomes. Such a care pathway incorporates local and national guidelines into everyday practice, to manage clinical risk and meet the requirements of clinical governance.
The NICE update on the treatment of schizophrenia recommends that general practitioners should take responsibility for organising physical health monitoring, including ECG and blood tests, and to share this information with psychiatrists for better patient care. 15,22 How the physical health of mentally ill populations will be monitored and delivered is an important topic for future research.
The prevalence of combination antipsychotic therapy reduced from 12% to 10% in 1 year between the two audit cycles. Similarly, the prevalence of high-dose antipsychotic treatment reduced from 3.7% (5 patients) to 2% (3 patients) in 1 year at re-audit. Patients who remained on high-dose antipsychotics at re-audit had treatment-resistant schizophrenia and an increased risk of violence and aggression. This subset of patients will always present therapeutic challenges and one could argue that guidelines developed for the majority of patients with schizophrenia are probably not appropriate and that more targeted guidance is required. This would of course be in keeping with current notions of a more personalised or ‘stratified’ treatment approach. Reference Taylor9
Although male patients were represented in both the HDAT and CAT groups, there were more males in the HDAT group than in the CAT group (74% v. 62%). This was reversed for female patients, with a relatively higher proportion of females in the CAT group than in the HDAT group (38% v. 26%). We could not explain the reason for this and recommend future research on this area.
As in previous studies, we found lack of compliance with available guidelines which may reflect difficulties in applying them in practice. Various difficulties in terms of staff time, resources, patients’ adherence to treatment and problems obtaining a second opinion were all reported as hindering compliance with guidelines. Reference Grech and Taylor8,Reference Howes, Vergunst, Gee, McGuire, Kapur and Taylor23,Reference Tyson, Mortimer and Wheeler25
Limitations of the audit
This was a retrospective study that used information available through PiMS and case notes. We did not look for details of use of the Mental Health Act 1983 or ethnicity (we had only a handful of patients treated under a compulsory treatment order and the population studied was predominantly White Scottish), and we were unable to obtain information on the concomitant use of other psychotropic medication, including discretionary antipsychotics and benzodiazepines, owing to a lack of clarity about daily dose and frequency of use of ‘as required’ medication. Patients in the community are reviewed less often and given that the use of ‘as required’ psychotropic medication is within the patient’s control, the dose is likely to vary from day to day depending on the mental well-being of individual patients. This is an important consideration and suggests that levels of combination and high-dose antipsychotic treatment are likely to be higher in practice than reported. This could be an important focus for future research.
Future recommendations
Regular audits within community services should be undertaken to drive improvements in practice as recommended by NICE guidance. National Health Service organisations have a responsibility to monitor adherence to combination and high-dose antipsychotic treatment monitoring protocols as part of clinical governance and cases of serious side-effects or mortality could give rise to litigation. Targeted, evidence-based prescribing using, where applicable, non-pharmacological interventions, may be the way forward to ensure patient safety and to improve quality of life in patients with a devastating and life-long condition such as schizophrenia.
Acknowledgements
We thank Professor Robert Hunter, consultant psychiatrist, Rehabilitation Unit, Gartnavel Royal Hospital and Clinical Director of the Psychiatric Research Institute of Neuroscience, PsyRING, NHS Greater Glasgow and Clyde Health Board, for constructive criticism and contribution in writing up this project, and Mrs Emma Banks for administrative support. We also thank Dr Rajeev Krishnadas, clinical lecturer, University of Glasgow and Sackler Institute of Psychobiological Research, Glasgow, and Dr David Young, senior lecturer, Department of Mathematics and Statistics, University of Strathclyde, Glasgow, for the statistical analysis of the data. We extend our thanks to all staff at the Arndale Mental Health Resource Centre for their encouragement and involvement, particularly Mrs Tina Cuthill for providing all necessary information.








eLetters
No eLetters have been published for this article.